While the performance of all engineering materials greatly depends on ever-present small concentrations of defects, new phenomena can develop if the defect concentration is strongly increased. For instance, studying and understanding metallic alloys and ceramics with a random placement of multiple atomic species on the crystal lattice could lead to improvements in the performance of lithium ion batteries and fuel cells, helping with waste energy recovery through thermoelectric energy conversion, and leading to better catalysts. They can also act as pinning centers in the macroscopic quantum state of high-temperature superconductors, strongly enhancing critical currents and also superconducting transition temperatures.
Disorder comes in many forms. At elevated temperatures, atoms vibrate strongly about their equilibrium positions, which impacts the way heat flows through the material.
Truly amorphous materials, in which the atomic positions themselves are disordered exhibit superior wear and corrosion resistance benefitting applications in thin film coatings, detectors, and optical waveguides. While a large amount of empirical knowledge and models exist about the many useful properties of amorphous materials and alloys, our understanding of how these properties originate from atomic scale configurations and interactions is still in its infancy.
Through a materials-by-design paradigm linking materials theory, simulation and novel synthesis and characterization methods, this research program seeks to understand, at an atomic level, how novel material properties can emerge as the direct consequence of disorder. We have identified three paradigmatic systems that each realize a form of thermal, chemical, or structural disorder:
- Thermal transport of disordered systems at high-temperature, where energy is carried not only by phonons but also electrons and photons
- High entropy oxide materials that contain randomly distributed mixtures of five or more chemical elements on a regular lattice;
- Amorphous optical coatings with exceptionally low mechanical loss for applications in gravitational wave detectors, optomechanics, and quantum information components.
In the first theme, simulations and characterization are being combined to explain the origins of the ‘heat trap’ phenomenon displayed by carbon nanotube forests discovered by our research teams.
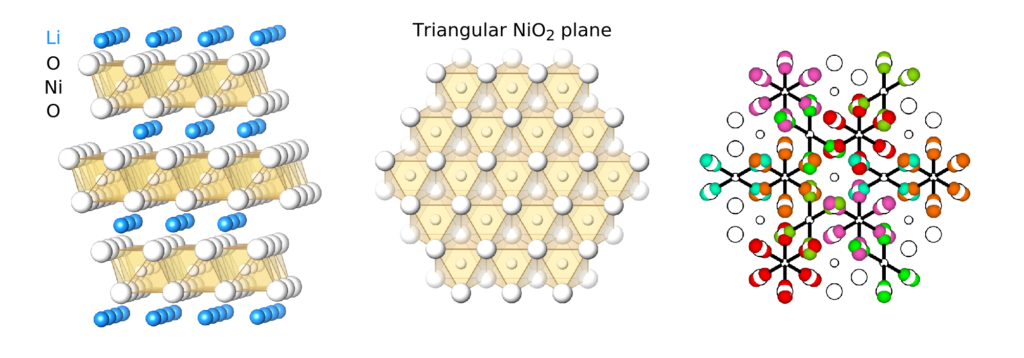
As part of the second theme, team members are studying the structure of lithium nickel oxide (LiNiO2), a precursor to a widely used lithium ion battery cathode material. In a recently published article, team members showed that LiNiO2 should be thought of as high-entropy glassy material instead of a crystalline ordered material. This novel perspective may pave the way for designing new cathode materials with improved performance.
In the third theme, Grand Challenge researchers are collaborating on the development of an improved mirror coating based on amorphous materials for LIGO (the Laser Interferometer Gravitational-Wave Observatory) along with Jess McIver from UBC’s Department of Physics & Astronomy. LIGO made headlines in 2016 for the experimental observation of gravitational waves and improved detector coatings that have the potential to dramatically increase the sensitivity to astronomical events of next-generation gravitational wave detectors.
Figure 1: Strong heat localization in carbon nanotubes

A figure showing a macroscopic-size carbon nanotube forest previously published in Physical Review B from the article, “Heat localization through reduced dimensionality” (Chang, Fan, Chowdhury, Sawatzky & Nojeh, 2018).
Description: A macroscopic-size carbon nanotube forest consisting of billions of millimetres-tall vertically aligned nanotubes. A low-power focused laser beam has illuminated a spot on the sidewall of the nanotube forest. Despite the conductive nature of nanotubes, the generated heat remains localized, forming a ‘Heat Trap’ and enabling efficient heating to very high temperatures at which strong black body radiation and thermionic emission take place. (The laser is infrared and not seen in the photo; the glow is due to localized incandescence.) This thermal confinement appears to have its origins in the low-dimensionality of the nanotube forest, and disorder and various forms of defects may also be playing an important role.
Figure 2: LiNiO2 as a high-entropy charge- and bond-disproportionated glass